Kinetochores coordinate pericentromeric cohesion and early DNA replication by cdc7-dbf4 kinase recruitment.
Toyoaki Natsume, Carolin A Müller, Yuki Katou, Renata Retkute, Marek Gierliński, Hiroyuki Araki, J Julian Blow, Katsuhiko Shirahige, Conrad A Nieduszynski, Tomoyuki U Tanaka
Mol. Cell (2013), 50(5):661-74![]() | PubMed | PubMed Central | Mol. Cell
| PubMed | PubMed Central | Mol. Cell
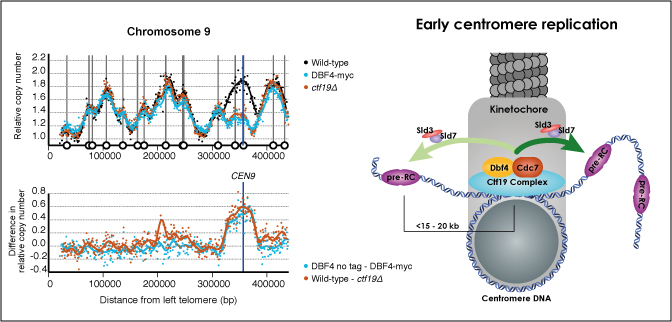
Centromeres play several important roles in ensuring proper chromosome segregation. Not only do they promote kinetochore assembly for microtubule attachment, but they also support robust sister chromatid cohesion at pericentromeres and facilitate replication of centromeric DNA early in S phase. However, it is still elusive how centromeres orchestrate all these functions at the same site. Here, we show that the budding yeast Dbf4-dependent kinase (DDK) accumulates at kinetochores in telophase, facilitated by the Ctf19 kinetochore complex. This promptly recruits Sld3-Sld7 replication initiator proteins to pericentromeric replication origins so that they initiate replication early in S phase. Furthermore, DDK at kinetochores independently recruits the Scc2-Scc4 cohesin loader to centromeres in G1 phase. This enhances cohesin loading and facilitates robust pericentromeric cohesion in S phase. Thus, we have found the central mechanism by which kinetochores orchestrate early S phase DNA replication and robust sister chromatid cohesion at microtubule attachment sites.
Our publications
Click to expand
2017
DNA replication timing influences gene expression level.
Müller & Nieduszynski (2017)
J. Cell Biol., 216(7):1907-1914
Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome.
Shen et al. (2017)
Science, 355(6329):
2015
Discovery of an Unconventional Centromere in Budding Yeast Redefines Evolution of Point Centromeres.
Kobayashi et al. (2015)
Curr. Biol., 25(15):2026-33
A global profile of replicative polymerase usage.
Daigaku et al. (2015)
Nat. Struct. Mol. Biol., 22(3):192-8
2014
The dynamics of genome replication using deep sequencing.
Müller et al. (2014)
Nucleic Acids Res., 42(1):e3
2013
High-resolution replication profiles define the stochastic nature of genome replication initiation and termination.
Hawkins et al. (2013)
Cell Rep, 5(4):1132-41
Accelerated growth in the absence of DNA replication origins.
Hawkins et al. (2013)
Nature, 503(7477):544-547
Stochastic association of neighboring replicons creates replication factories in budding yeast.
Saner et al. (2013)
J. Cell Biol., 202(7):1001-1012
A Link between ORC-origin binding mechanisms and origin activation time revealed in budding yeast.
Hoggard et al. (2013)
PLoS Genet., 9(9):e1003798
Replisome stall events have shaped the distribution of replication origins in the genomes of yeasts.
Newman et al. (2013)
Nucleic Acids Res., 41(21):9705-18
Avoiding chromosome pathology when replication forks collide.
Rudolph et al. (2013)
Nature, 500(7464):608-11
Kinetochores coordinate pericentromeric cohesion and early DNA replication by cdc7-dbf4 kinase recruitment.
Natsume et al. (2013)
Mol. Cell, 50(5):661-74
High quality de novo sequencing and assembly of the Saccharomyces arboricolus genome.
Liti et al. (2013)
BMC Genomics, 14():69
2012
Mathematical modeling of genome replication.
Retkute et al. (2012)
Phys Rev E Stat Nonlin Soft Matter Phys, 86(3 Pt 1):031916
Conservation of replication timing reveals global and local regulation of replication origin activity.
Müller & Nieduszynski (2012)
Genome Res., 22(10):1953-62
A putative homologue of CDC20/CDH1 in the malaria parasite is essential for male gamete development.
Guttery et al. (2012)
PLoS Pathog., 8(2):e1002554
2011
OriDB, the DNA replication origin database updated and extended.
Siow et al. (2011)
Nucleic Acids Res., 40(Database issue):D682-6
Dynamics of DNA replication in yeast.
Retkute et al. (2011)
Phys. Rev. Lett., 107(6):068103
Comparative functional genomics of the fission yeasts.
Rhind et al. (2011)
Science, 332(6032):930-6
From sequence to function: Insights from natural variation in budding yeasts.
Nieduszynski & Liti (2011)
Biochim. Biophys. Acta, 1810(10):959-66
2010
Mathematical modelling of whole chromosome replication.
de Moura et al. (2010)
Nucleic Acids Res., 38(17):5623-33
2009
The origin recognition complex interacts with a subset of metabolic genes tightly linked to origins of replication.
Shor et al. (2009)
PLoS Genet., 5(12):e1000755
Detection of replication origins using comparative genomics and recombinational ARS assay.
Nieduszynski & Donaldson (2009)
Methods Mol. Biol., 521():295-313
2008
Analysis of chromosome III replicators reveals an unusual structure for the ARS318 silencer origin and a conserved WTW sequence within the origin recognition complex binding site.
Chang et al. (2008)
Mol. Cell. Biol., 28(16):5071-81
2006
OriDB: a DNA replication origin database.
Nieduszynski et al. (2006)
Nucleic Acids Res., 35(Database issue):D40-6
Genome-wide identification of replication origins in yeast by comparative genomics.
Nieduszynski et al. (2006)
Genes Dev., 20(14):1874-9
2005
The requirement of yeast replication origins for pre-replication complex proteins is modulated by transcription.
Nieduszynski et al. (2005)
Nucleic Acids Res., 33(8):2410-20
2004
The cyclin A1-CDK2 complex regulates DNA double-strand break repair.
Müller-Tidow et al. (2004)
Mol. Cell. Biol., 24(20):8917-28
Cyclin A1 protein shows haplo-insufficiency for normal fertility in male mice.
van der Meer et al. (2004)
Reproduction, 127(4):503-11
2002
Whole-genome analysis of animal A- and B-type cyclins.
Nieduszynski et al. (2002)
Genome Biol., 3(12):RESEARCH0070
Ku complex controls the replication time of DNA in telomere regions.
Cosgrove et al. (2002)
Genes Dev., 16(19):2485-90